Pre-optimized for the ScanLater™ Western Blot Detection Cartridge, this assay kit enables the first western blot detection capability in a microplate reader. Utilizing this substrate-free assay, researchers can achieve equivalent sensitivity to traditional chemiluminescence and fluorescence-based assays, while consolidating western blot and ELISA applications onto a single reader.
- Overview
- Data
- Technology
- Resources
The ScanLater™ Western Blot Assay Kit is a time-resolved fluorescence (TRF)-based assay optimal for quantitating as little as femtogram protein samples. Using this novel western blot method, researchers can:
- Eliminate time-dependent substrate addition steps
- Sustain blot signal stability for at least one month
- Enhance assay sensitivity using TRF method to reduce background noise
- Maintain femtogram to picogram protein sensitivity similar to traditional western blot detectors
Features
- Time-resolved fluorescence detection
- Europium-labeled goat anti-rabbit, goat anti-mouse, donkey anti-rat, donkey anti-goat or streptavidin labeled antibodies
- Supports nitrocellulose and PVDF membranes
- 340/80 nm excitation with 616/10 nm emission range
- Optimized for use with ScanLater Western Blot Detection cartridge for the SpectraMax® i3 and Paradigm® Multi-Mode Platforms
Representative applications:
- Protein identification and quantitation
- Epitope mapping
- Amino acid composition and sequence analysis
- Spots imprinting
- Phosphoprotein endogenous or exogenous expression
- Protein resilience
- Structure domain analysis
- Protein expression
- Protein content in serum
- QC elimination of albumin and LgG in serum
- Protein expression the the cell cycle
Signal stability over time
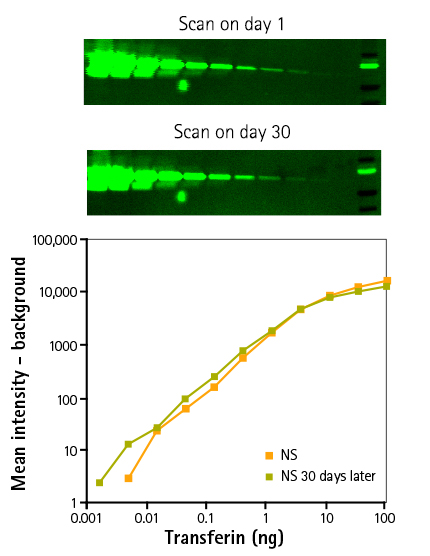
Figure 1: Results demonstrate the signal stability of the ScanLater Assay System. Three dilutions of Transferrin were separated by electrophoresis and transferred to an Immobilon FL membrane and probed with rabbit anti-transferrin. Blots were incubated with the ScanLater Eu-labeled Anti-rabbit Secondary Antibodies. Membranes were measured on day 1 and day 30. Results represent a three-fold serial dilution of transferrin 1x sample buffer was loaded on a 4-20% gradient gel run for 30 min. Proteins were transferred to an Immobilon FL membrane and probed with rabbit anti-transferrin overnight at 4˚C followed by incubation with europium-labeled anti-rabbit IgG for 1 hr. Blots were washed, dried, and scanned on the same day and after a month using a SpectraMax Paradigm Reader.
Signal stability across multiple scans
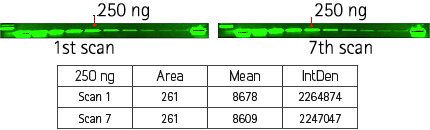
Figure 2: Results demonstrate the signal stability of the ScanLater Eu-labeled Secondary Antibodies after 7 successive measurements of the same membrane. Demonstrates stability of europium-labeled anti-rabbit IgG blots after 7 scans on the SpectraMax Paradigm Reader.
Detection of trace amounts of endogenous phosphorylated form of pChk1
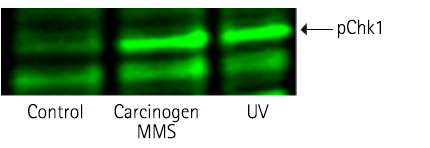
Figure 3: Detection of phosphorylated form of pChk1 in HEK 293 cells on treatment with carcinogen MMS & UV radiation (Stanford University).
Detection of trace amounts of endogenous phosphorylated form of JNK1

Figure 4: Detection of phosphorylated form of JNK1 in NHLF (natural human lung fibroblast) cells treated with stimulants & inhibitory drugs.
Comparison of ScanLater Western Blot System to chemiluminescent western blot detection
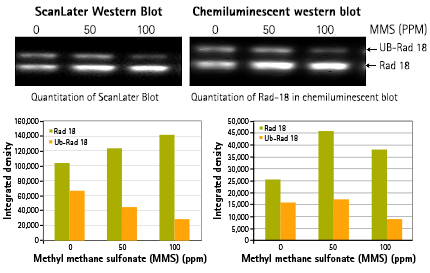
Figure 5: Detection of ubiquitinated Rad-18 in HEK 293 cells on treatment with different concentration (0, 50, 100 ppm) of carcinogen MMS, an alkylating agent. Comparable result using ScanLater Western Blot System vs. chemiluminescence western blot (Stanford University).
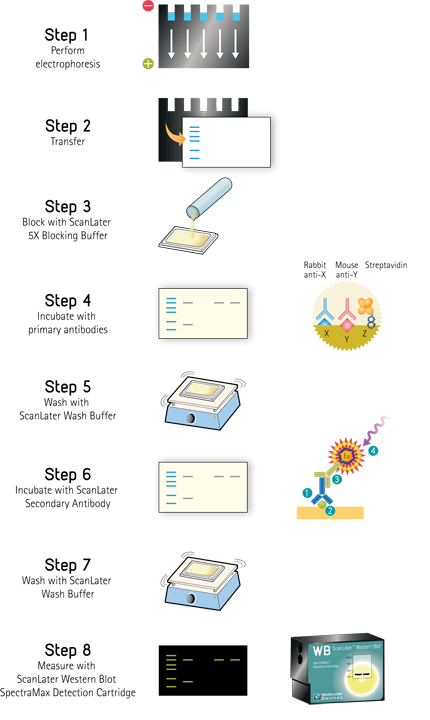
ScanLater Western Blot Assay Workflow
ScanLater Western Blot Assay Principle
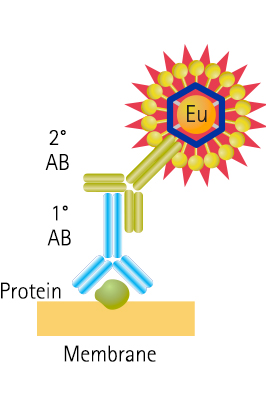
Figure 2: Schematic of protein detection method shows the secondary antibody is directly labeled with Europium.
Europium-chelate excitation and emission spectra
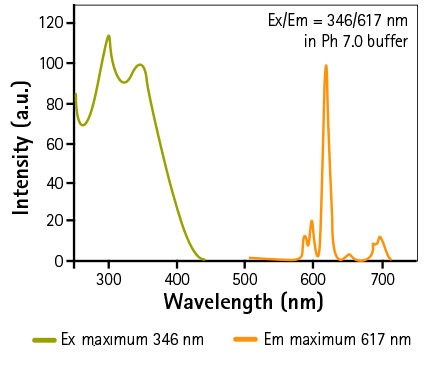
Figure 3: Schematic representation of Europium-chelate excitation and emission spectra
Emission lifetimes of background fluorescence
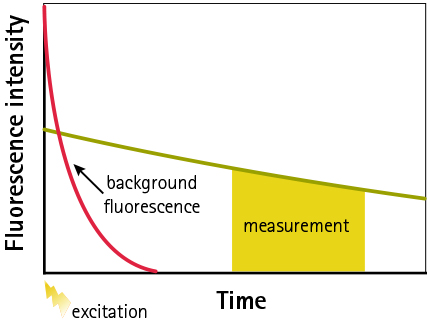
Figure 4: Schematic representation of emission lifetimes of background fluorescence and Eu-chelate showing principle of TRF measurement. Signal is measured after appropriate time-delay to reduce auto-fluorescence.
