酵素 - IMAP評価キット
酵素 - IMAP評価キットとは
IMAP®テクノロジーは、基質ペプチドの配列に関係なく、様々なキナーゼ、ホスファターゼ、ホスホジエステラーゼに適用可能なホモジニアスアッセイを提供します。このアッセイは、混合して読み取るだけの簡単な手順で、酵素活性を正確に測定することができます。
・IMAPは、キナーゼ、ホスファターゼ、ホスホジエステラーゼをスクリーニングするための完全なアッセイシステムを提供する。
・IMAPアッセイは抗体ベースではないので、汎用性があり、あらゆるキナーゼ、ホスファターゼ、ホスホジエステラーゼに使用できる。
・ロバスト性の蛍光シグナルにより、信頼性の高い結果が得られ、Zファクターも良好である。
・IMAPアッセイはホモジニアスであり、コスト削減のための小型化が可能である。
・IMAPアッセイは、FPおよびTR-FRET検出モードの両方が利用可能で、ユーザーのスクリーニングニーズに対応する。
酵素 - IMAP評価キット
ホスホ基と3価の金属含有ナノ粒子(ビーズ)との特異的で親和性の高い相互作用に基づくIMAPは、キナーゼ、ホスファターゼ、ホスホジエステラーゼ活性を評価するための汎用的な非抗体ベースのプラットフォームである。蛍光標識した基質を用いて酵素反応を行う。IMAP Binding Systemを添加すると酵素反応が停止し、ビーズとリン酸化基質との結合が始まる。酵素活性に相関する基質のビーズへの結合は、読み出しとしてFPまたはTR-FRETを用いて検出することができる。
IMAPプログレッシブバインディングシステム
IMAP Progressive Binding Systemにより、研究者は使用する基質ごとにFPおよびTR-FRETアッセイ条件を最適化できる。このシステムはProgressive Binding Buffer AとProgressive Binding Buffer B、およびProgressive Binding Reagentで構成されている。2つのバッファーと試薬は、選択する基質の酸性特性、望ましいATP濃度、バックグラウンドパラメーターに応じて、異なる割合で組み合わせることができる。
IMAP基質
モレキュラーデバイスは、検証済みの基質およびキャリブレーターを幅広く提供しています。基質は、本来バリデートされた酵素と共に使用することも、他の酵素の基質として使用することもできます。
・最適化された結合条件を持つ検証済みのIMAP基質は、IMAPプラットフォームの使用時に最高のパフォーマンスを保証します。
・基質には2種類のサイズがあり、IMAP FPとIMAP TR-FRETの両方で使用可能
・ 数十種類の基質が100種類以上の酵素で検証されています。多くの基質が赤または緑の蛍光ラベルで利用可能で、マルチプレックスを可能にし、化合物の自家蛍光から生じる問題に対処する方法を提供する。
酵素 - IMAP評価キットのデータ
-
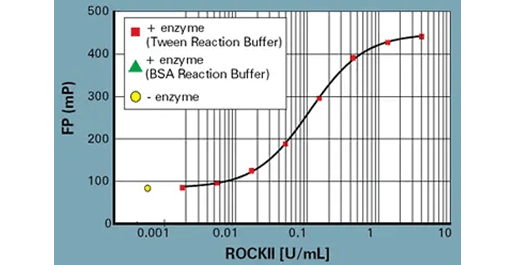
サンプルIMAP FPデータ: ROCKII希釈曲線
Progressive Binding Systemを用いたBSAおよびTween IMAP Reaction Buffer中のROCKIIの酵素希釈曲線。反応条件 ROCKIIキナーゼ(Upstate: 14-451)、100 nM FAM-S6由来基質(5FAM-AKRRRLSSLRA-COOH、R7184)、100 µM ATP。IMAP結合溶液の条件: 100% Binding Buffer A、Progressive Binding Reagent 1:400。 -
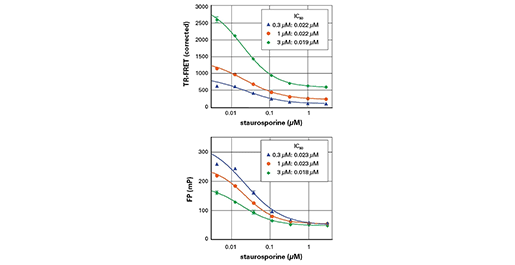
FP対TR-FRET検出
スタウロスポリンによるAkt阻害: IMAP TR-FRET検出(上)とIMAP FP検出(下)の比較。反応条件: Aktキナーゼ(0.1u/ml、Upstate:14-276)、300 nM、1µM、3µM FAM-Crosstide(5FAM-GRPRTSSFAEG-COOH、R7110)、10 µM ATP、スタウロスポリンは指示通り。IMAP結合溶液: 40% Binding Buffer A、60% Binding Buffer B、Progressive Binding Reagent 1:400。
酵素 - IMAP評価キットの技術
-
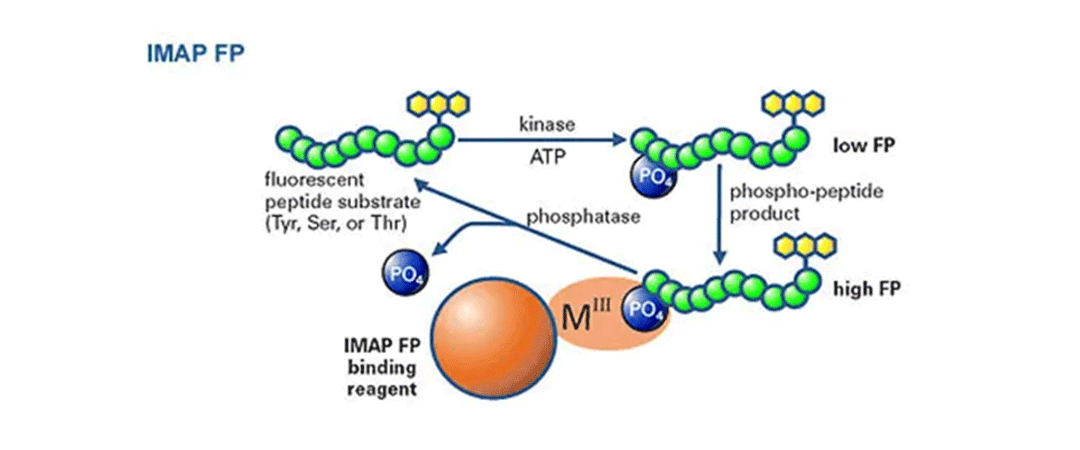
IMAP FP キナーゼおよびホスファターゼ汎用アッセイ
FP読み出しを用いたIMAPの原理: 蛍光標識ペプチドを用いたキナーゼ反応後に結合液を添加する。リン酸化された小さな蛍光基質は、大きなM(III)ベースのナノ粒子に結合し、基質の回転速度を低下させ、蛍光偏光を増加させる。 -
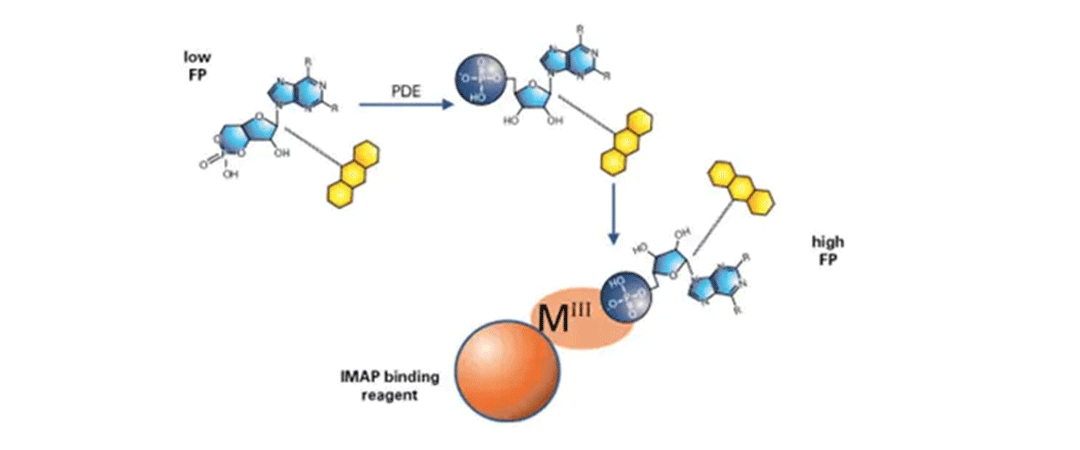
IMAP FP汎用ホスホジエステラーゼアッセイ
FP読み出しを用いたIMAP原理: 蛍光標識基質を用いたホスホジエステラーゼ反応後に結合液を添加する。リン酸化された小さな蛍光基質は、大きなM(III)ベースのナノ粒子に結合し、基質の回転速度を低下させ、蛍光偏光を増加させる。 -
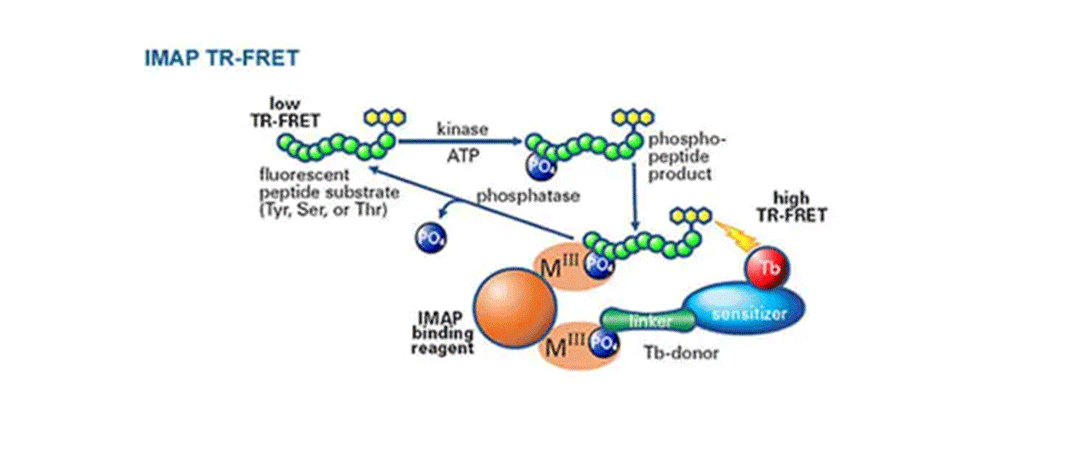
IMAP TR-FRET 一般的なキナーゼおよびホスファターゼアッセイ
TR-FRET読み出しを用いたIMAPの原理: 蛍光標識ペプチドまたはタンパク質を用いたキナーゼ反応後に、結合溶液を添加する。このシステムでは、ナノ粒子にテルビウム(Tb)-ドナー分子をスパイクする。スパイクされたM(III)ベースのナノ粒子に結合することで、リン酸化された蛍光基質がTb-Donorに近接し、Tb-Donorとリン酸化された蛍光基質との間のTR-FRETを測定することができる。 -

IMAP TR-FRET 汎用ホスホジエステラーゼアッセイ
TR-FRET読み出しを用いたIMAPの原理: 蛍光標識基質を用いたホスホジエステラーゼ反応の後に、結合溶液を添加する。このシステムでは、ナノ粒子にテルビウム(Tb)-ドナー分子をスパイクする。スパイクされたM(III)ベースのナノ粒子に結合することで、リン酸化された蛍光基質がTb-Donorに近接し、Tb-Donorとリン酸化された蛍光基質との間のTR-FRETを測定することができる。
酵素 - IMAP評価キットに対応する製品・サービス
-
FlexStation 3 マルチモードマイクロプレートリーダー

イオンチャネル・GPCR活性の測定に威力を発揮する
自動マルチチャンネルピペッター搭載マルチマイクロプレートリーダー -
SpectraMax Paradigm マルチモードマイクロプレートリーダー

高速で設定可能なマイクロプレートリーダー1台でハイスループットスクリーニングが可能
-
SpectraMax i3x マルチモードマイクロプレートリーダー

研究ニーズに合わせて進化できるマイクロプレートリーダー
-
SpectraMax Mシリーズ マルチモードマイクロプレートリーダー

妥協ない性能と低価格を両立したマルチモードマイクロプレートリーダー