FLIPRカリウムチャネル測定キット
FLIPRカリウムチャネル測定キットとは
FLIPR® カリウムチャネル測定キットは、リガンドおよび電位依存性カリウムチャネルの機能活性を測定します。ホモジニアスで洗浄不要のアッセイプロトコルは、大きなシグナルウィンドウと高いZ'値を提供します。
FLIPRカリウムチャネル測定キット
- ・細胞ベースアッセイにおけるカリウムチャネル活性の機能的測定
- ・ホモジニアス無洗浄プロトコールにより、ウェル間のばらつきを低減し、ワークフローを簡素化
- ・非ホモジニアスアッセイと比較してシグナルウィンドウを拡大
- ・迅速なプロトコールで作業時間を短縮
FLIPR カリウムチャネル測定キットは、電位依存性およびリガンド依存性のカリウム(K+)チャネルを介したタリウムイオン(Tl+)の透過性を利用します。このアッセイでは、新規の高感度Tl+インジケーター色素が利用され、カリウムチャネルを通してTl+に結合すると明るい蛍光シグナルを発する。Tl+シグナルの強度は、開口状態にあるカリウム・チャネルの数に比例するため、カリウム・チャネル活性の機能的な指標となる。さらに、シグナル対SN比を向上させるために、当社特許取得済みのマスキング色素のひとつを使用して、バックグラウンド蛍光をさらに減少させる。
FLIPRカリウムチャネル測定キットのデータ
-
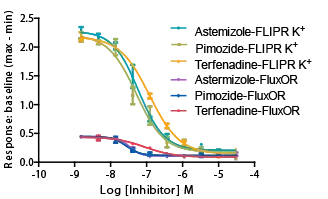
カリウムキットの比較
FLIPR カリウムチャネル測定キットと他社キットの結果の比較。 -
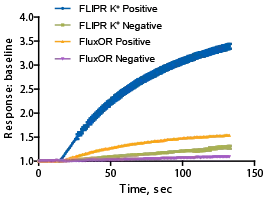
シグナルウィンドウの比較
FLIPR カリウムチャネル測定キットと競合キットのシグナルダイナミックレンジの比較。ネガティブコントロールは4μMテルフェナジン、ポジティブコントロールはバッファー。FLIPR カリウムチャネル測定キットのZ'ファクター=0.85、n=32に対し、競合キットのZ'ファクター=0.64、n=30。 -
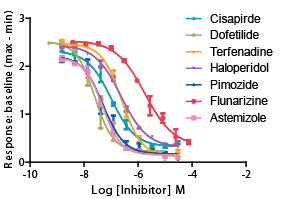
hERGチャネル阻害
参考化合物によるhERGチャネルの濃度依存的阻害。 -
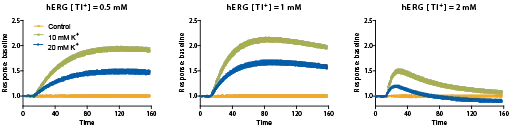
hERGチャネル刺激剤の最適化
細胞を色素とともにインキュベートし、FLIPR Tetra システムで検出中に刺激剤バッファーを添加した。異なる条件下で、シグナルの濃度依存的な応答を特徴付けた。最適なシグナルは、1 mM Tl+と10 mM K+(最終濃度)の刺激剤バッファーを無塩化物バッファーで希釈した組み合わせで得られた。
FLIPRカリウムチャネル測定キットの技術
-
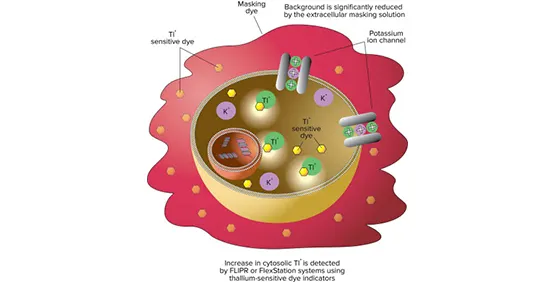
アッセイキットのメカニズム
このアッセイは、カリウム(K+)チャネルに対するタリウム(Tl+)の透過性を利用する。細胞を蛍光性タリウム感受性色素のAMエステル体とインキュベートする。細胞内のエステラーゼがAM基を切断し、色素を細胞内に閉じ込める。次にセルは、K+とTl+の混合液、またはTl+存在下のリガンドで刺激される。蛍光シグナルの増加は、カリウムチャネルを介して特異的にTl+が細胞内に流入したことを表し、カリウムチャネル機能活性の指標となる。バックグラウンドはマスキング溶液によって減少する。
FLIPRカリウムチャネル測定キットに対応する製品・サービス
-
SpectraMax iD3/iD5 マルチモードマイクロプレートリーダー

大型タッチスクリーンを備えた高感度マイクロプレートリーダー
-
FlexStation 3 マルチモードマイクロプレートリーダー

イオンチャネル・GPCR活性の測定に威力を発揮する
自動マルチチャンネルピペッター搭載マルチマイクロプレートリーダー -
FLIPR Penta ハイスループットセルベーススクリーニングシステム

リード化合物の同定および化合物の安全性評価を目的とした
ハイスループットカイネティックスクリーニングに理想的なシステム