Detects compound activity against known and/or orphan GPCR targets in a high content screening environment
The Transfluor® Assay is a cell-based fluorescence assay used to screen for G-protein coupled receptor (GPCR) ligands and other potential drugs that regulate GPCRs by deactivating or desensitizing a common pathway. By attaching a fluorescent label to beta-arrestin, the location of the receptor-arrestin complex may be monitored during receptor activation. Since desensitization only occurs with an activated receptor, monitoring beta-arrestin translocation and subsequent receptor recycling provides a method to detect the activation of any GPCR.
- Overview
- Technology
- Data
- Resources
Studying this mechanism enables researchers to identify the impact of GPCR ligands on a variety of physiological processes including behavior, mood, inflammation, nervous system, and olfactory responses. In addition, researchers can gain insight into the complexities of receptor degradation and recycling for a given target.
Features
- Validated with over 100 GPCRs, the Transfluor assay provides the assurance to work across all GPCR classes (Class I, II, III), regardless of interacting G-protein (Gs, Gi/o and Gq/11).
- Single read-out is compatible with all GPCR subtypes, including Gi, Gs, Go, Gq, eliminating the need for multiple GPCR assays
- No GPCR labeling or tagging avoids custom molecular biology that may alter your target’s response.
- Universal format requires no prior knowledge of interacting G-protein, enabling orphan GPCR screening.
- GFP-based technology does not require additional substrates to monitor beta-arrestin translocation.
- MetaXpress® Application Module automates analysis of beta-arrestin movement to the cell membrane (pits) and endocytic vesicles.
- Ligand Independent Translocation (LITe™) Assay is an agonist-independent assay used to verify the translocation of beta-arrestin-GFP in orphan GPCRs.
Orphan GPCR Assay
In contrast to current methods of screening GPCRs, the Transfluor technology is based on the mechanism for termination of GPCR signaling, known as receptor desensitization. This mechanism is shared by virtually all GPCRs and is activated by ligand binding. Transfluor technology requires no prior knowledge of the interacting G-protein. This important feature of the Transfluor technology makes it ideal for screening orphan GPCRs (oGPCR).
A proprietary technique to assist in validating orphan GPCR screens, called LITe™ (Ligand Independent Translocation), is an agonist-independent assay used to verify the translocation of ß arrestin-GFP in orphan GPCRs.
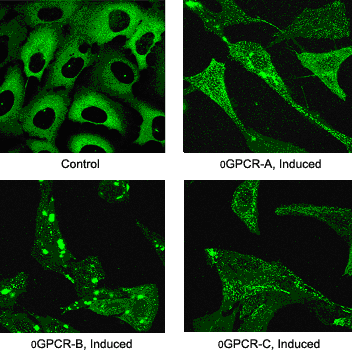
Transfluor References
Garippa RJ, Hoffman AF, Gradl G, Kirsch A.
High-throughput confocal microscopy for beta-arrestin-green fluorescent protein translocation G protein-coupled receptor assays using the Evotec Opera.
Methods Enzymol. 2006;414:99-120.
Hudson CC, Oakley RH, Sjaastad MD, Loomis CR.
High-content screening of known G protein-coupled receptors by arrestin translocation.
Methods Enzymol. 2006;414:63-78.
Oakley RH, Hudson CC, Sjaastad MD, Loomis CR.
The ligand-independent translocation assay: an enabling technology for screening orphan G protein-coupled receptors by arrestin recruitment.
Methods Enzymol. 2006;414:50-63.
Haasen D, Wolff M, Valler MJ, Heilker R.
Comparison of G-protein coupled receptor desensitization-related beta-arrestin redistribution using confocal and non-confocal imaging.
Comb Chem High Throughput Screen. 2006 Jan;9(1):37-47.
Ghosh RN, DeBiasio R, Hudson CC, Ramer ER, Cowan CL, Oakley RH.
Quantitative cell-based high-content screening for vasopressin receptor agonists using transfluor technology.
J Biomol Screen. 2005 Aug;10(5):476-84.
Ozawa K, Hudson CC, Wille KR, Karaki S, Oakley RH.
Development and validation of algorithms for measuring G-protein coupled receptor activation in cells using the LSC-based imaging cytometer platform.
Cytometry A. 2005 May;65(1):69-76.
Oakley RH, Hudson CC, Cruickshank RD, Meyers DM, Payne RE Jr, Rhem SM, Loomis CR.
The cellular distribution of fluorescently labeled arrestins provides a robust, sensitive, and universal assay for screening G protein-coupled receptors.
Assay Drug Dev Technol. 2002 Nov;1(1 Pt 1):21-30.
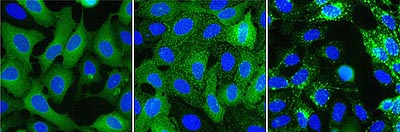
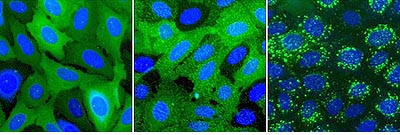
Figure 1.ß2AR-expressing cells were stimulated with isoproterenol. Left: control, center: pits, right: vesicles.
Top: Transfluor assay imaged with ImageXpress. Bottom: Transfluor assay imaged with Discovery-1.
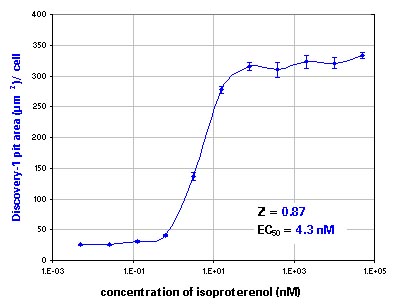
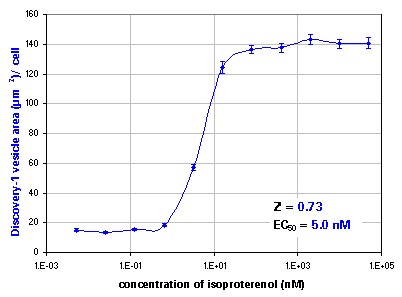
Figure 2.Segmentation and analysis run with the GPCR Cycling Application Module for MetaXpress.
Dose response to isoproterenol. Top: pits, bottom: vesicles. Error bars show standard error.
Partial GPCR Listing
The Transfluor technology monitors receptor activity by detecting movement of beta-arrestin-GFP in the cell.A partial listing of GPCRs that have been shown to translocate beta-arrestin-GFP is shown below.
| Gs | Gi/o | Gq/11 |
|
|
|
- 12 Drosophila GPCRs
- Fz4 frizzled receptor
- TßRIII transforming growth factor-ß
