シングルチャネルから大電流記録まで
Axon Instrumentsの増幅器シリーズは、パッチクランプ実験の全範囲における最高クラスの機器です。増幅器のシリーズには低ノイズでシングルチャンネル記録用 AxopatchTM200B、ホールセルボルテージクランプとハイスピードカレントクランプ記録用 MultiClampTM700B、また2電極ボルテージクランプとカレントクランプ記録用 AxoclampTM900Aがあります。-

最小のS/N比
Axopatch200Bキャパシタフィードバックパッチクランプ増幅器は、革新的なキャパシターフィードバック技術により、低ノイズのシングルチャネル記録を実現しています。 -

複数の実験に対応
MultiClamp700B微小電極増幅器は、ホールセルボルテージクランプとカレントクランプ記録を可能にしています。シリーズの中では一番多目的な増幅器です。 -

大電流の実験に対応
Axoclamp900A微小電極増幅器は、高出力への対応が可能で、大電流のボルテージクランプとカレントクランプ記録を促進します。

特徴
 データシート
データシートAxon Digidata 1550B Plus HumSilencer
Axon Digidata 1550Bハムサイレンサー付き低ノイズデーター取得システムの詳細こちらから
Download Data Sheet
 データシート
データシートAxon Axoclamp 900A微小電極用増幅器
Axon Axoclamp 900A微小電極用増幅器のデータシートはこちらから。この機器はシングルセル、細胞組織のスライスからのシグナルを測定するいくつかの操作モードがあります。
Download Data Sheet
アクソンインストメント パッチクランプ増幅器アプリケーション
-
シングルチャネル記録
Tパッチクランプ法はガラスマイクロピペットが細胞膜にギガオームシール(GΩ)形成することが必要となります。そのマイクロピペットはイオンを通す為、電解質の溶液に浸かったワイヤーも必要になります。ギガオームシールを作成後、膜の「パッチ」が細胞から引っ張られます。もし、そのパッチの中にシングルイオンチャネルがあれば、電流を測定することができます。超低ノイズでとても小さなコンダクタンスを持つイオンチャネルにAxopatch200Bは理想的です。 -
イオンチャネル
イオンチャネルは脂質二重層に気孔を形成している蛋白質の集まりです。それぞれのチャネルは特定のイオンだけを通します(例:カリウム, ナトリウム、カルシウム、塩化物)。パッチクランプはとても感度の良い増幅器、質の高いデータ取得装置、結果を評価する便利なソフトウェアを使い、イオンチャネルの活動と関連する膜の中の電流や電圧をリルタイムで直接測定するのに使われています。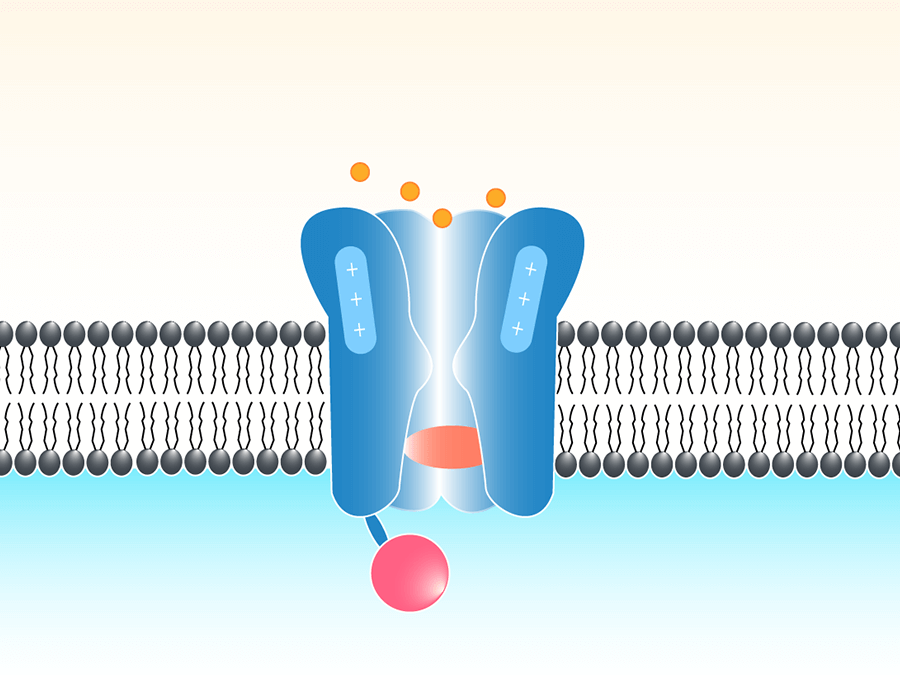
-
パッチクランプ
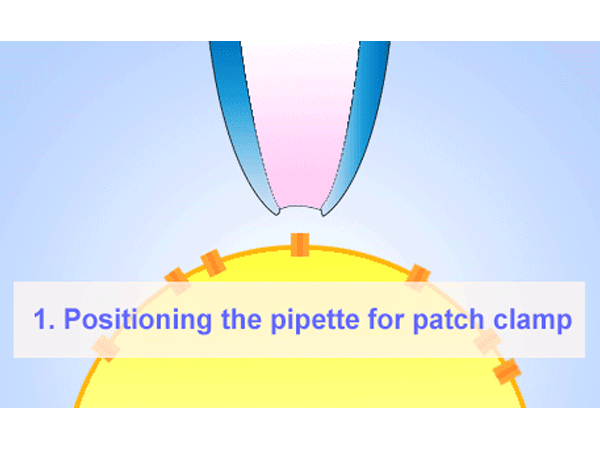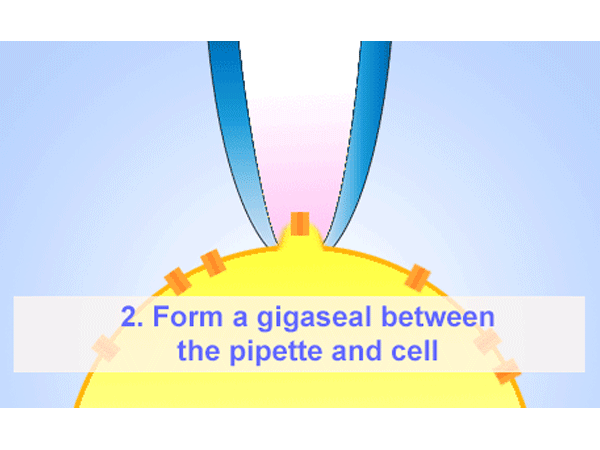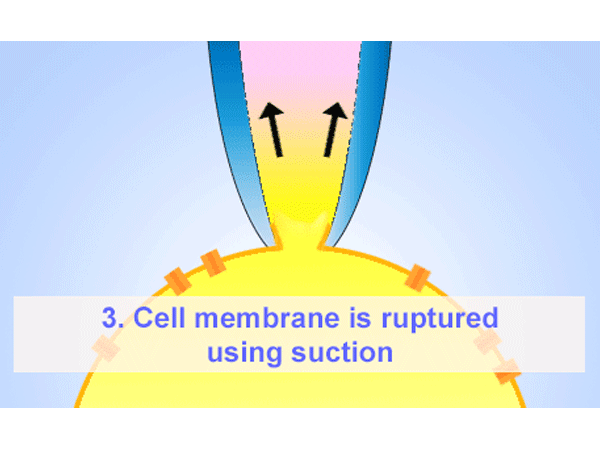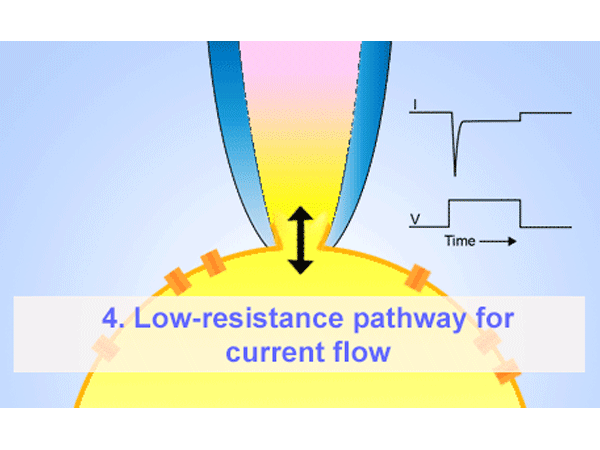
パッチクランプ法はガラスマイクロピペットが細胞膜にギガオームシール(GΩ)形成することが必要となります。そのマイクロピペットはイオンを通す為、電解質の溶液に浸かったワイヤーも必要になります。ホールセル記録法は低アクセス抵抗を提供する為、膜電圧をコントロールし、膜のパッチを弱めの吸引で破ります。その他に、インサイドーアウトまたはアウトサイド-アウト法を使い、膜のパッチを細胞から引っ張りシングルチャネルを通る電流を測定することもできます。 -
ホールセル記録
ホールセルパッチクランプ法はガラスマイクロピペットが細胞膜にギガオームシール(GΩ)形成することが必要となります。そのマイクロピペットはイオンを通す為、電解質の溶液に浸かったワイヤーも必要になります。弱めの吸引によって膜のパッチは破られ細胞全体へ低抵抗でアクセスできます。それにより、膜を通る電圧をコントロールし、膜を通ってイオンチャネルを通る電流を測ることができます。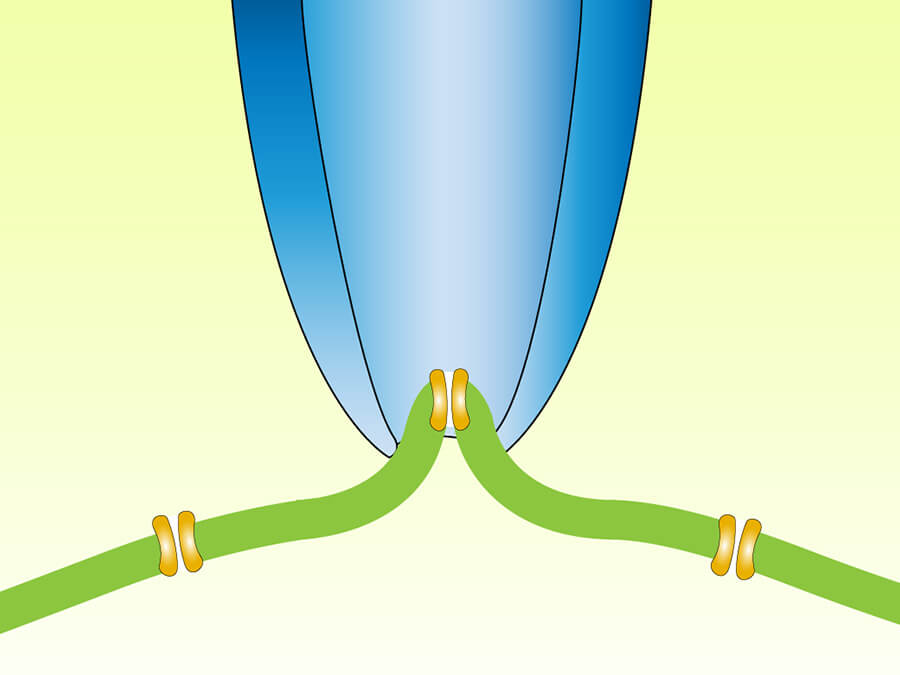
-
シリーズレジスタンス補正
シリーズレジスタンスは増幅器とホールセル記録法を使用する細胞内との間のすべての抵抗の合計です。オームの法則により、この抵抗が大きければ大きいほど、コマンドレベルと計測された値の差が大きくなります。これにより実際に電圧、または電流測定にエラーが生じる可能性があります。この問題を克服する為、モレキュラーデバイスの増幅器はシリーズレジスタンスへの電圧、電流降下で起こるエラーを補正する回路を組み込んであります。 -
ボルテージクランプ増幅器
ボルテージクランプ法を実験で使用する時、細胞内の膜電位をコントロールし、その電圧を維持するのに必要な電流を測定します。この電圧コントロールをコマンドボルテージと呼びます。このコマンドボルテージを維持するには増幅器は電流を流す必要があります。流された電流は開いているイオンチャネル電流と同じ、また逆になります。それにより増幅器は開いているイオンチャネルの膜を流れる電流の量を測ることを可能にしています。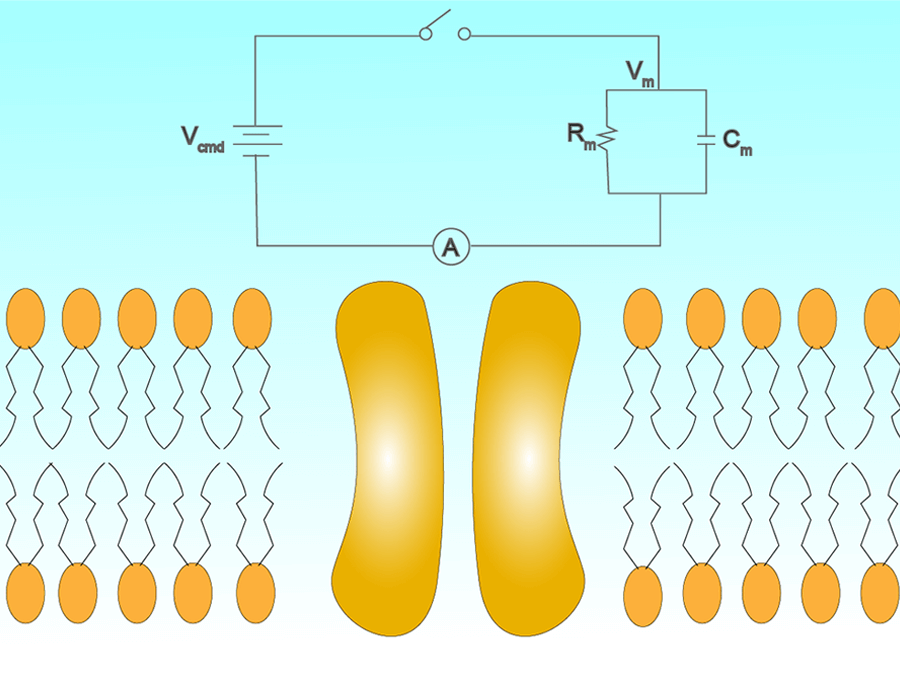
-
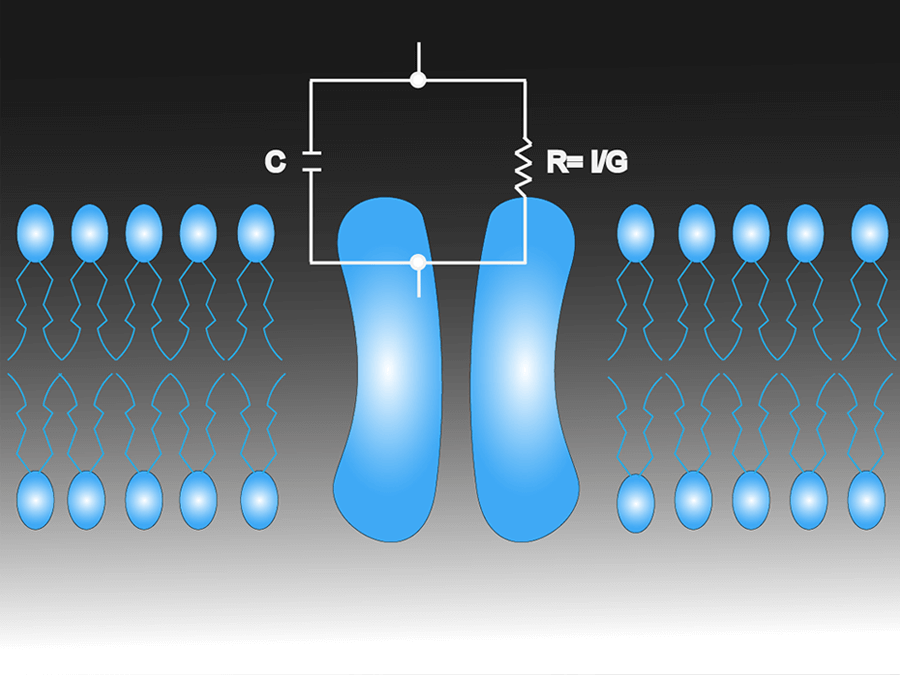
カレントクランプ増幅器
カレントクランプは電流注入して膜電位を測定する方法です。膜電位を測るには、MultiClamp700BとAxoclamp900Aは、電流注入による電圧降下をモニターします。カレントクランプ法は一般に模擬刺激ですが、本物のような波形を細胞に加え、細胞膜への影響を観察します。この方法は活動電位などの重要な細胞的活動の評価に適しています。
Specifications & Options of Axon Instruments Patch-Clamp Amplifiers
Axopatch™ 200B Capacitor Feedback Patch Clamp Amplifier |
MultiClamp™ 700B Microelectrode Amplifier |
Axoclamp™ 900A Microelectrode Amplifier |
|
| 仕様 | |||
| アプリケーション |
シングルチャネル記録、ホールセルパッチクランプ記録、細胞外記録、アンペロメントリー/ボルタンメントリー、人工細胞膜、ナノポア研究 |
シングルチャネル記録、ホールセルパッチクランプ記録、細胞内微小電極記録、細胞外記録、アンペロメントリー/ボルタンメントリー、人工細胞膜、ナノポア研究 |
ホールセルカレントクランプ記録、細胞内微小電極記録、細胞外記録、2電極ボルテージクランプ記録 |
| スマートテレグラフ |
ゲイン、フィルター、キャパシタンス |
ゲイン、フィルター、キャパシタンス、入出力スケーリングファクター、記録モード- |
ゲイン、フィルター、キャパシタンス、入出力スケーリングファクター、記録モード |
| シールテスト |
|
|
|
| ホールディング電圧範囲 |
-/+ 1000 mV |
-/+ 1000 mV |
-/+ 200 mV for dSEVC and TEVC modes |
| ホールディング電流範囲 |
-/+ 200 nA |
-/+ 200 nA |
Up to -/+ 1/Ro (feedback resistor) |
| 出力ゲイン (mV/pA) |
0.5 to 500 |
1.0 to 2000 |
1.0 to 2000 |
| モード |
I-Clamp, V-Clamp |
I-Clamp, V-Clamp |
I-Clamp, DCC, HVIC, dSEVC, TEVC |
| オープン回路での5kHsと10kHzのノイズ |
0.06 pA rms, (5 kHz), 0.13 pA rms (10 kHz) |
0.15 pA rms, (5 kHz), 0.28 pA rms (10 kHz) |
|
| ボルテージクランプレジスタンスフィードバック |
True for for cSEVC |
True for for cSEVC |
True for for dSEVC |
| 容量補正 |
|
|
|
| RMS ノイズ |
0.13 pA rms (10 kHz) increases to only 0.145 pA RMS when a patch-pipette holder is attached |
0.28 pA rms (10 kHz) at 50 GΩ to 3.0 pA rms (10 kHz) at 50 MΩ |
20 mV at 10 kHz |
| 自動オシレーション抑制 |
|
|
|
| カレントクランプとボルテージクランプの自動モード変更 |
|
|
|
| 電位保持用のスローカレントインジェクション |
|
|
|
| カレントクランプ専用回路 |
|
|
|
| デユアルパッチクランプ記録 |
|
|
|
| コンピューターコントロール* |
|
|
|
| 一般的なインターフェース |
|
|
|
Axon Instruments パッチクランプ増幅器 リソース
 データシート
データシートAxon Digidata 1550B Plus HumSilencer
Axon Digidata 1550Bハムサイレンサー付き低ノイズデーター取得システムの詳細こちらから
Download Data Sheet データシート
データシートAxon Axoclamp 900A微小電極用増幅器
Axon Axoclamp 900A微小電極用増幅器のデータシートはこちらから。この機器はシングルセル、細胞組織のスライスからのシグナルを測定するいくつかの操作モードがあります。
Download Data Sheet データシート
データシートAxon Axopatch 200B 微小電極用増幅器
超低ノイズを実現する冷却機能を備えたAxon Axopatcch 200B微小電極増幅器の詳細はこちらから
Download Data Sheet
 Data Sheet
Data SheetMultiClamp 700B Microelectrode Amplifier
Learn about MultiClamp 700B Amplifier for electrophysiology and electrochemistry which is capable of single-channel & whole-cell voltage patch clamp, and much more.
Download Data Sheet
 プレゼンテーション
プレゼンテーションデータ取得用Clampex, MultiClamp, digitizerの接続設置
データ取得し、自分の実験に必要性な設定を理解できるようになります。
Download Presentations
Videos and Demos









Ready to get started?
We are ready to help you solve your tough research challenges. Our proven solutions and highly qualified teams across the globe can help advance your next big discovery.







